A week of global new drug research and development progress
The following article is from Yaodu Daily, and the author was published by Yaodu Daily
 Yaodu Daily.
Yaodu Daily.
Yaodu Daily, focusing on the latest progress in domestic pharmaceutical research and development, is your one-stop drug research and development information assistant! Yao Du Xiao D, see you every day!
Part 1
Global drug approval/research and development trends
01 Global new drug approvals
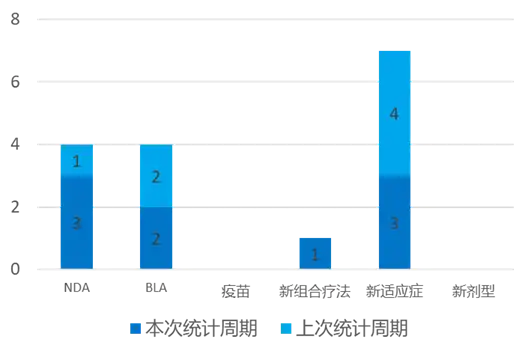
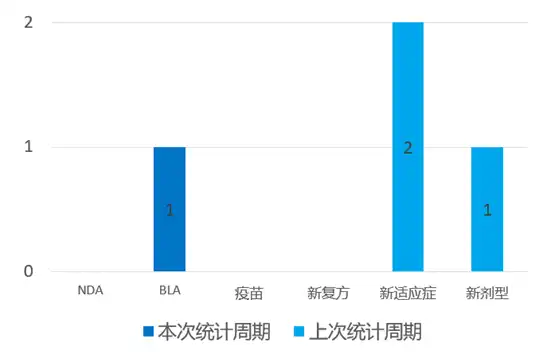
According to the statistical analysis of drug transition data, a total of 9 new drugs have been approved for marketing globally (excluding China) during this statistical period (2024.03.16 - 03.22). Among them, 3 were approved by the NDA, 2 were approved by the BLA, 1 was approved for new combination therapies, and 3 were approved for new indications. Compared with the previous statistical cycle, 2 new drugs have been approved this time.
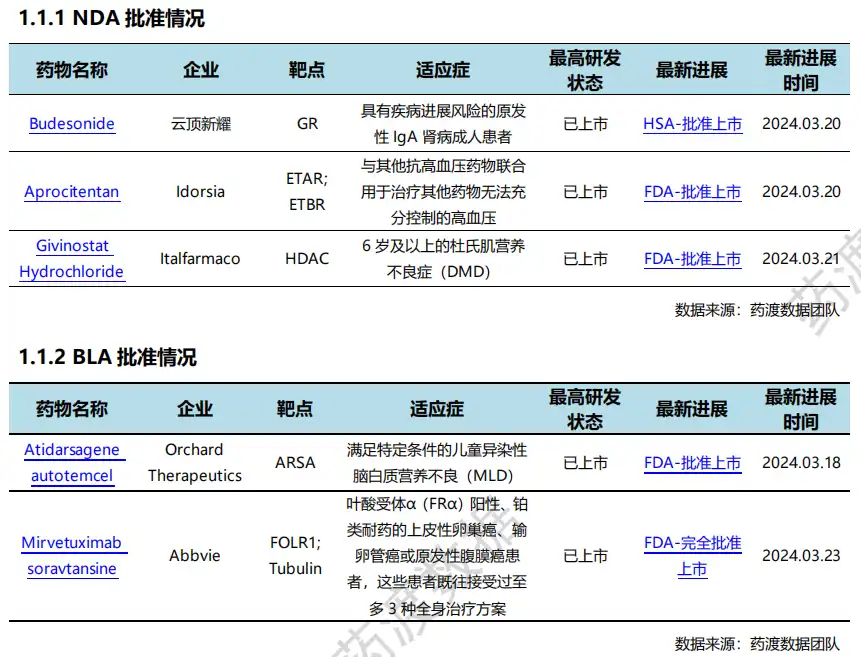
 3月20日,Idorsia 宣布FDA已批准TRYVIO™(Aprocitentan)与其他抗高血压药物联合用于治疗其他药物未得到充分控制的成年患者的高血压。TRYVIO (Aprocitentan) 是一种内皮素受体拮抗剂,可抑制内皮素(ET)-1与ET的结合一个和 ETB受体,在TRYVIO获得批准之前,没有针对ET通路的全身性抗高血压药物。TRYVIO在美国的获批标志着Idorsia的又一个重要里程碑。 3月22日,FDA完全批准ELAHERE®(Mirvetuximab soravtansine- gynx)用于治疗叶酸受体α(FRα)阳性、铂类耐药上皮卵巢癌、输卵管癌或原发性腹膜成人癌症患者,这些患者既往接受过多达三种治疗。ELAHERE的完全批准是基于验证性MIRASOL的III期试验,该试验支持该药物作为叶酸受体α(FRα)阳性铂类耐药卵巢癌(PROC)的潜在新标准。数据显示,ELAHERE治疗将癌症进展的风险降低了35%。
3月20日,Idorsia 宣布FDA已批准TRYVIO™(Aprocitentan)与其他抗高血压药物联合用于治疗其他药物未得到充分控制的成年患者的高血压。TRYVIO (Aprocitentan) 是一种内皮素受体拮抗剂,可抑制内皮素(ET)-1与ET的结合一个和 ETB受体,在TRYVIO获得批准之前,没有针对ET通路的全身性抗高血压药物。TRYVIO在美国的获批标志着Idorsia的又一个重要里程碑。 3月22日,FDA完全批准ELAHERE®(Mirvetuximab soravtansine- gynx)用于治疗叶酸受体α(FRα)阳性、铂类耐药上皮卵巢癌、输卵管癌或原发性腹膜成人癌症患者,这些患者既往接受过多达三种治疗。ELAHERE的完全批准是基于验证性MIRASOL的III期试验,该试验支持该药物作为叶酸受体α(FRα)阳性铂类耐药卵巢癌(PROC)的潜在新标准。数据显示,ELAHERE治疗将癌症进展的风险降低了35%。
Global (excluding China) new drug approvals (partial)
02 Progress in global new drug application
According to the statistical analysis of drug transition data, there were 2 new drugs submitted for marketing worldwide (excluding China) during this statistical period (2024.03.16 - 03.22). Among them, 1 NDA application progress and 1 BLA application progress. This NDA/BLA application progress has increased by one compared with the previous statistical cycle.
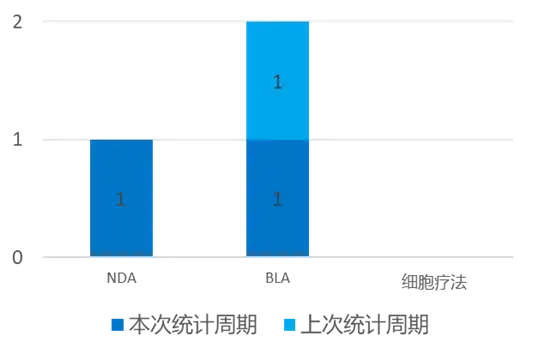
On March 21, Novo Nordisk announced that Awiqli had received marketing authorization. Awiqli is a weekly basal insulin analogue. The CHMP has taken positive advice and recommended Awiqli for the treatment of adult diabetes. The positive opinion of the CHMP is based on the results of the ONWARDS Phase 3a clinical trial program. Novo Nordisk expects to receive final marketing authorization from the European Commission in approximately two months.
NDA/BLA application status
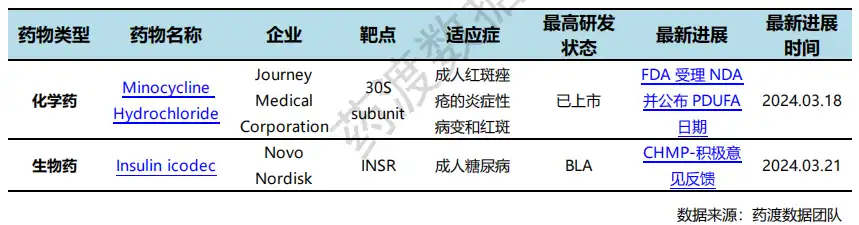
According to the statistical analysis of drug transit data, a total of 3 drugs worldwide (excluding China) have been specially qualified by regulatory agencies during this statistical period (2024.03.16 - 03.22). Among them, 2 are chemical drugs and 1 is cell therapy. Compared with the previous statistical cycle, there are 5 fewer drugs that have been specially qualified by regulatory agencies in this statistical cycle.
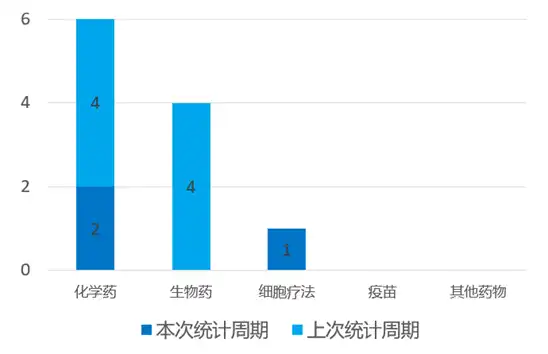
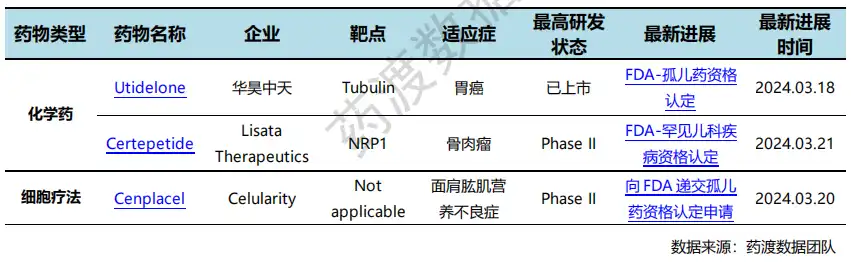
On March 18, the FDA awarded Huahao Zhongtian Youtidelong Oral Capsule orphan drug qualification for the treatment of gastric cancer based on excellent data demonstrated by a two-phase, multi-center Phase II clinical study. As of February 1, 2024, a total of 11 patients have completed the efficacy evaluation, of which 8 patients have achieved PR and 3 patients have achieved SD, with a clinical benefit rate of 100%! Utederon has stronger activity, a broader anti-cancer spectrum, no obvious hematological toxicity, is not easy to develop drug resistance, is still effective against multidrug resistant tumors such as taxus, can penetrate the blood-brain barrier, and is environmentally friendly for fermentation and production of genetically engineered bacteria., and can be administered orally. Currently, Utederon is conducting simultaneous clinical trials in China and the United States. Preliminary data shows that its bioavailability is as high as 57%. It also has the advantages of low effective dose, wide treatment window, and less toxic and side effects.
Special qualifications recognition
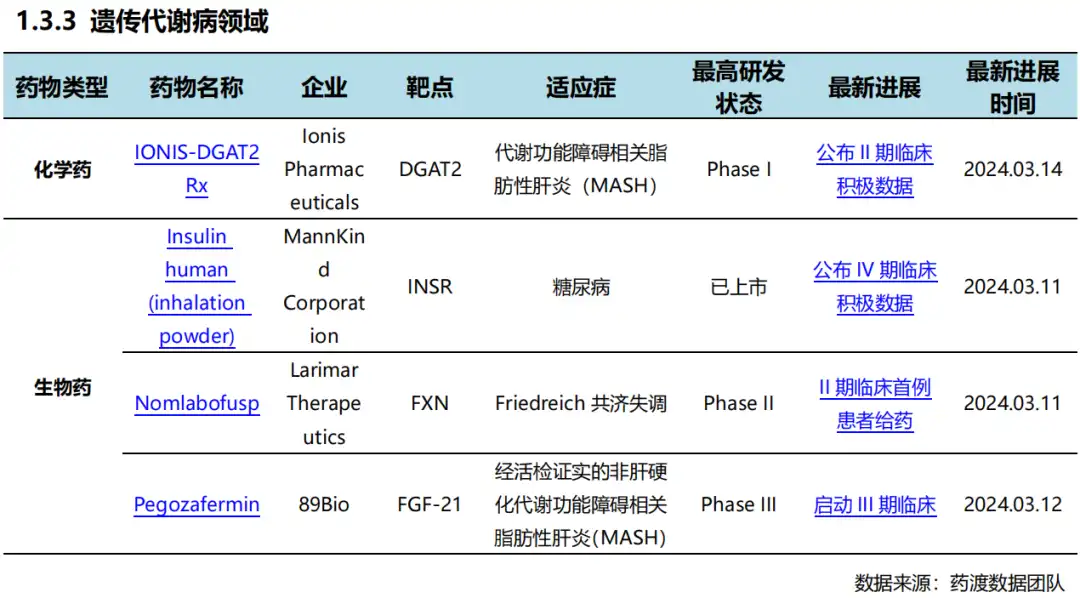
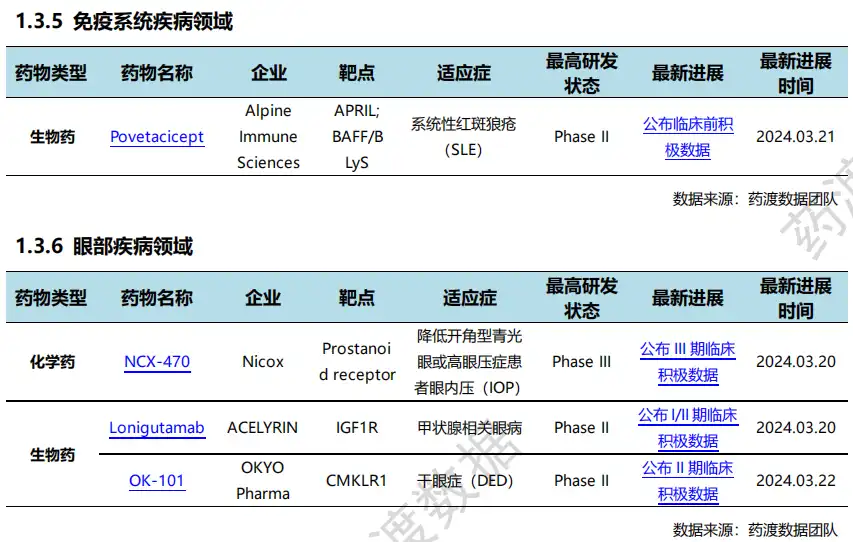
03 Progress in global new drug research and development 根据药渡数据统计分析,本次统计周期(2024.03.16-03.22)全球(不含中国)新药临床研发状态更新共计26条,涉及肿瘤、遗传代谢病呼、精神疾病、免疫系统疾病以及皮肤病等共计11个领域。
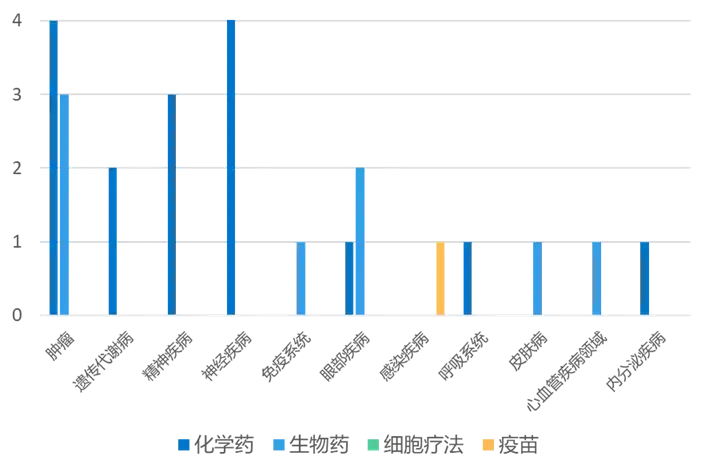
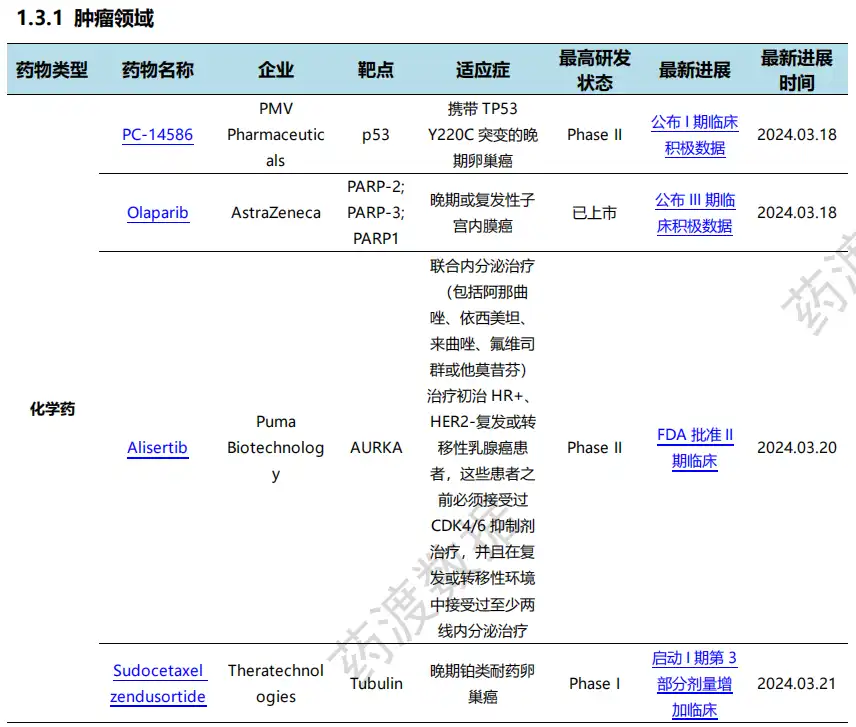
Among them, the update of clinical progress in the field of oncology ranks first in all fields, involving 4 chemical drugs and 3 biological drugs.
On March 16, GlaxoSmithKline announced that in the RUBY/ENGOT-EN6/GOG3031/NSGO Phase III trial, the overall survival (OS) results in Part 1 and the progression-free survival (PFS) results in Part 2 were statistically and clinically significant in adult patients with primary advanced or recurrent endometrial cancer. FDA regulatory applications based on RUBY Part 1 data will be accepted in the first half of this year to expand indications for the overall population. RUBY Part 2 data showed that adding niraparib to maintenance treatment significantly improved PFS in first-line primary advanced or recurrent endometrial cancer compared with chemotherapy alone, meeting the trial's primary endpoint.
On March 21, Bionomics reported the results of a complete dataset analysis of the ATTUNE Phase IIb trial of BNC210 in patients with post-traumatic stress disorder, and will meet with the FDA at the end of the second quarter of 2024 to discuss the registration plan for BNC210 in PTSD, with a late trial expected to be launched by the end of 2024.
The efficacy results of the ATTUNE Phase IIb trial met its primary endpoint and several secondary endpoints, highlighting the potential of BNC210 to address multiple key symptoms experienced by patients with PTSD. Effects were observed as early as week 4, supporting the potential for rapid onset of clinical efficacy consistent with the mechanism of action of BNC210. BNC210 continues to show psychoactive characteristics of non-sedation, non-habit formation, and non-cognitive impairment in psychoactive experimental therapy, and has good safety profile.
Details of global new drug research and development progress (part)

04 Global pharmaceutical transaction incident
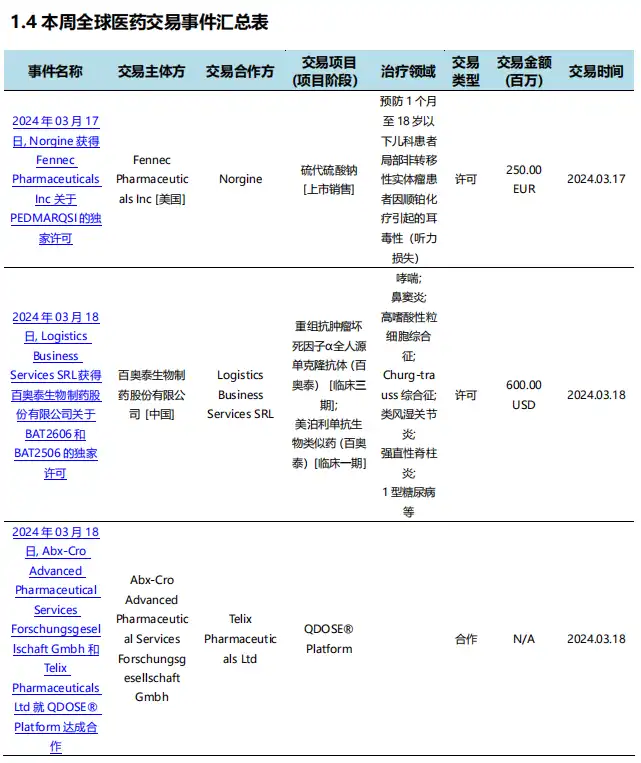
During this statistical period (2024.03.16 - 03.22), there were a total of 28 pharmaceutical transactions around the world (including China), involving many transaction events such as drug equity transfers and company mergers and acquisitions.
Summary of Global Pharmaceutical Transaction Hours (Part)
Part 2
Domestic drug approval/research and development trends
01 Domestic new drug approvals 根据药渡数据统计分析,本次统计周期(2024.03.16-03.22)国内共有1个新药获NMPA批准上市。其中, BLA批准1个。与上次统计周期相比,本次减少2个NMPA批准新药。
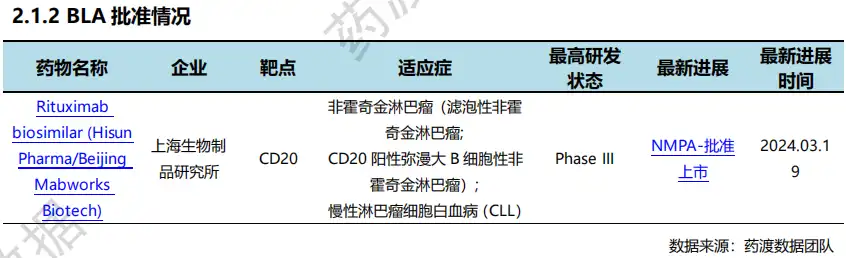
On March 19, the first antibody drug rituximab injection (trade name) developed by Shanghai Institute of Biological Products of China Biotech:Shenglijian ®) has obtained the Drug Registration Certificate approved and issued by NMPA. Shenglijian ® is approved for the treatment of non-Hodgkin lymphoma (follicular non-Hodgkin lymphoma, CD20-positive diffuse large B-cell non-Hodgkin lymphoma), chronic lymphoma cell leukemia (CLL) indications. This approval for listing marks the completion of the entire chain of antibody drug research and development and transformation at the Shanghai Institute of Biological Products.
Based on pharmaceutical comparisons and preclinical studies, the Shanghai Institute of Biological Products conducted head-to-head comparative studies of Phase I clinical trials and Phase III clinical trials. The marketing approval of Shenglijian ® is based on a multi-center, randomized, double-blind, positive parallel-controlled Phase III clinical study to compare Shenglijian ® combined with CHOP regimen and the original comparator drug combined with CHOP regimen in the treatment of previously treated patients with CD20-positive diffuse large B-cell lymphoma. The dose of the experimental drug Shenglijian ® and the original control drug was both 375mg/m2/BSA, intravenously instilled for 6 cycles of treatment. Clinical statistical results showed that the two groups of clinical patients had similar efficacy, safety, immunogenicity and pharmacokinetic characteristics. Domestic new drug approvals (partial)
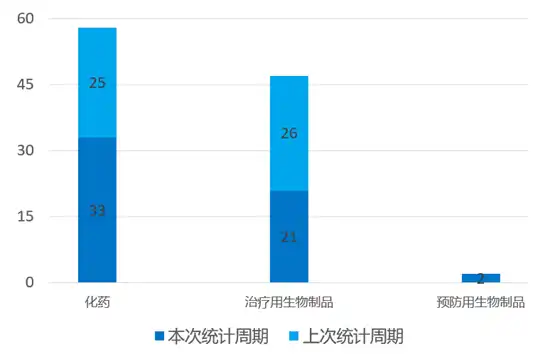
02国内新药临床默示许可进展 根据药渡数据统计分析,本次统计周期(2024.03.16-03.22)国内共有56个新药获临床默示许可,涉及85个受理号。其中,化学药33个,治疗用生物制品21个。与上个统计周期相比,本次增加10个临床默示许可获批受理号。
Progress on implied clinical approval of new drugs in China this week (partial)
03国内新药申报进展
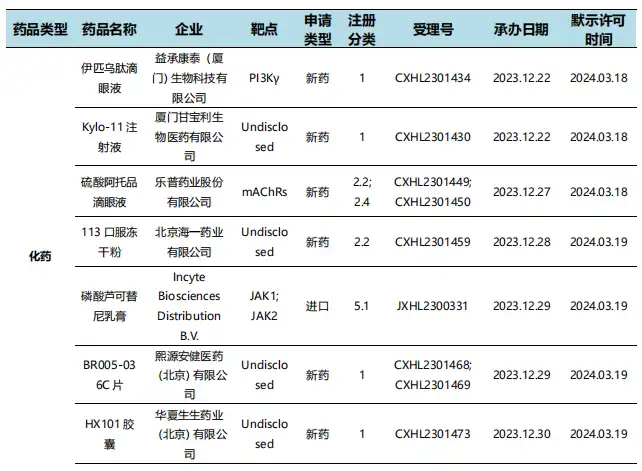
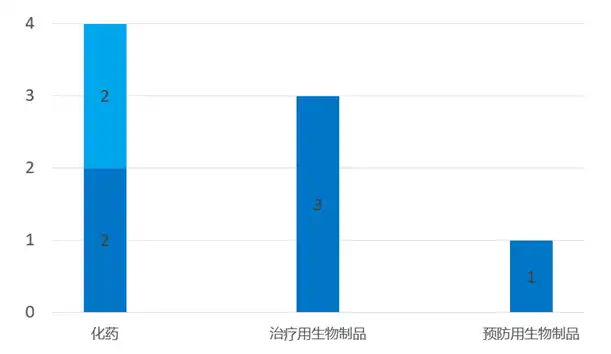
According to the statistical analysis of drug transit data, there were 6 new drugs submitted for marketing in China during this statistical period (2024.03.16 - 03.22), involving 12 acceptance numbers. Among them, 2 are chemical drugs, 3 are therapeutic biological products, and 1 is preventive biological product. Compared with the previous statistical cycle, 9 new listing application acceptance numbers have been added this time.
Domestic new drug application and marketing status (partial)
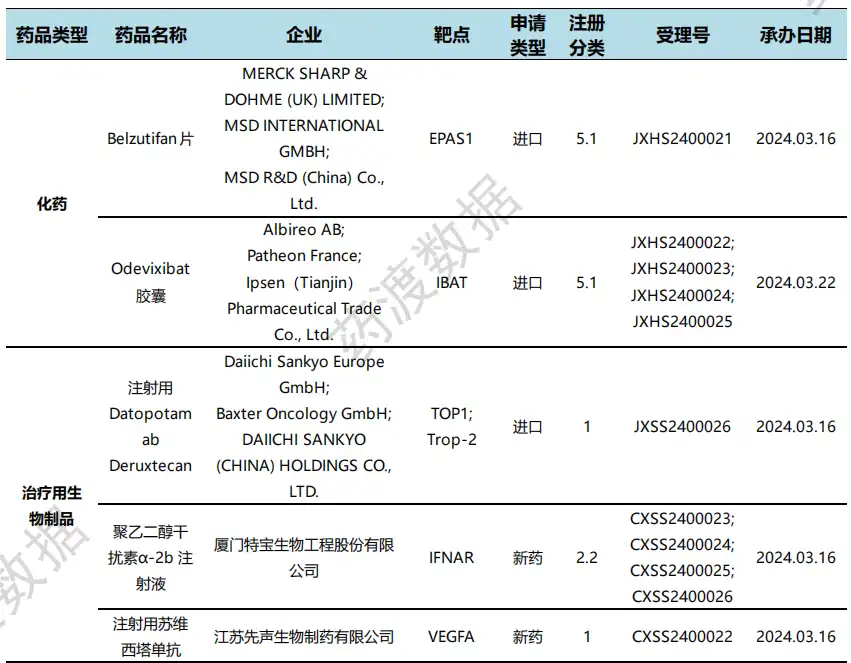
According to the statistical analysis of drug transition data, there were 22 clinical applications for new drugs in China during this statistical period (2024.03.16 - 03.22), involving 31 acceptance numbers. Among them, 9 are chemical drugs, 12 are therapeutic biological products, and 1 is preventive biological product. Compared with the previous statistical cycle, 11 clinical application acceptance numbers have been reduced this time.
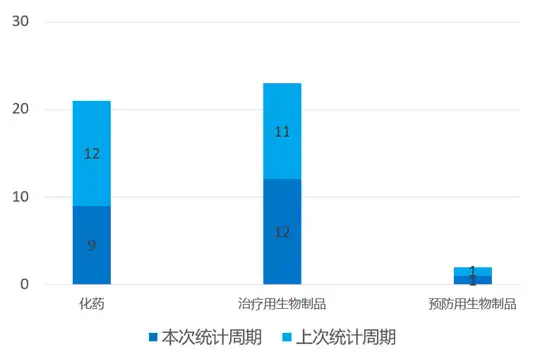
Domestic new drug clinical application status (partial)
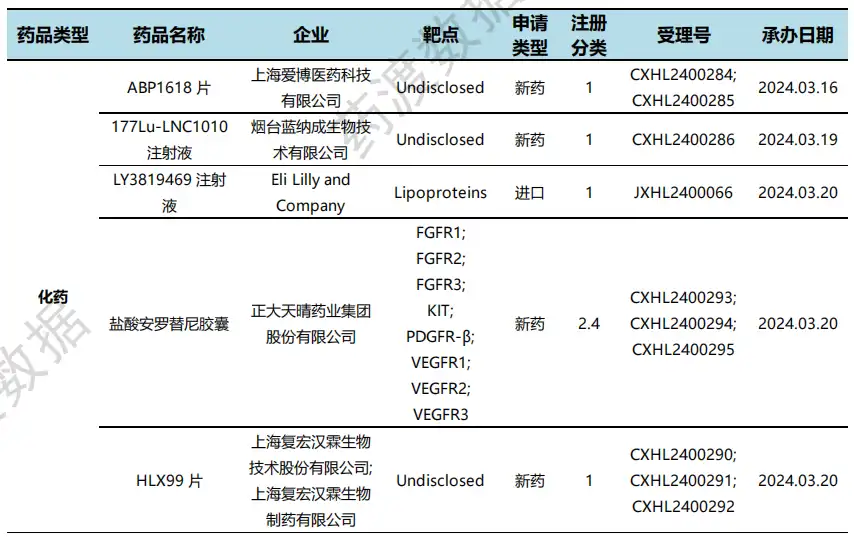
According to the statistical analysis of drug transit data, a total of 2 drugs in China have been recognized as special qualified by NMPA during this statistical period (2024.03.16 - 03.22). Among them, 1 is a chemical drug and 1 is a biological drug. Compared with the previous statistical cycle, there is one less drug in this statistical cycle that has been specially qualified by regulatory agencies.
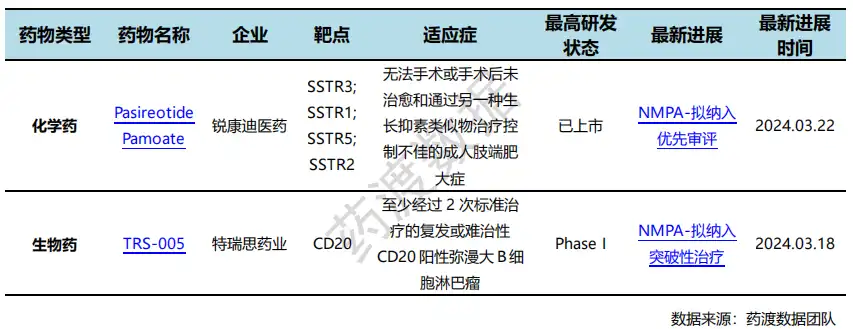
On March 22, the official website of CDE announced that the pareotide pamoate microspheres for injection submitted by Rekandi Pharmaceutical will be included in the priority review, and the proposed indications are: Treat adult patients with acromegaly who are inoperable or not cured after surgery and who are not well controlled with another somatostatin analogue. This is a long-acting pasireotide pamoate. The overall goal of treating acromegaly is to inhibit GH oversecretion, reduce IGF-1 levels, reduce complications, and reduce mortality. In 2014, pareotide pamoate was approved by the European Medicines Agency (EMA) and the US FDA for the treatment of adult acromegaly.
On March 18, CDE proposed Trith's TRS005 for injection into a breakthrough treatment product, with the indication for relapsed or refractory CD20-positive diffuse large B-cell lymphoma that has undergone at least 2 sessions of standard treatment. TRS005 has shown good efficacy in the Phase I clinical stage:The overall ORR for the 5 evaluable patients was 42.2%, with a CR of 11.1%; the ORR in the 0.5, 1.0, 1.5 and 1.8 mg/Kg dose groups were 42.9%, 33.3%, 43.8%, and 50.0%, respectively. The 1.8 mg/Kg dose group had the highest ORR (CR of 14.3%, PR of 35.7%) and DCR (85.7%).
NMPA special qualification recognition
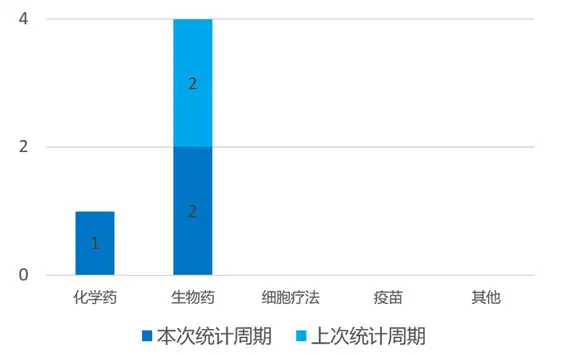
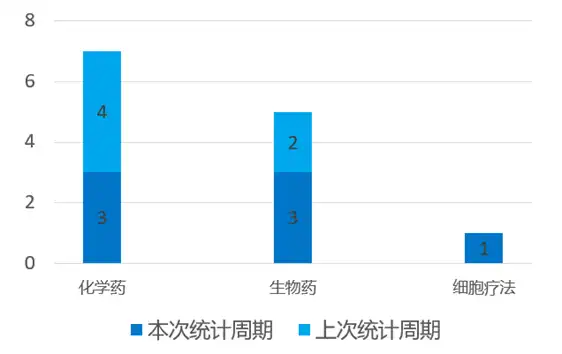
04国内新药研发进展 根据药渡数据统计分析,本次统计周期(2024.03.16-03.22)国内新药临床研发状态更新共计7条,涉及肿瘤、呼吸系统疾病、皮肤病、心血管疾病等共计6个领域。其中,化学药3个,生物药3个,疫苗1个。
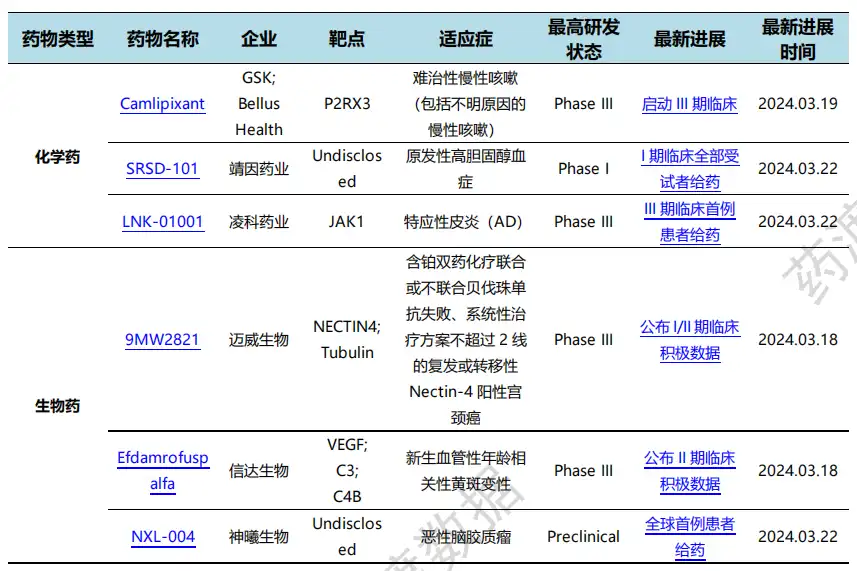
On March 16, Maiwei Biotech reported clinical research data on 9MW2821 cervical cancer at the annual meeting of the American Society of Gynecologic Oncology (SGO). 9MW2821 is the first global drug with the same target to report clinical data in the indication of cervical cancer. The data released this time demonstrate its effectiveness and good safety in the field of cervical cancer, and is expected to bring new breakthroughs in the treatment of recurrent or metastatic cervical cancer and meet a large number of unmet clinical needs.
On March 19, Suzhou Xinda Biotech in Rockville, USA announced that its second Phase II clinical study of IBI302 (high-dose) in Chinese subjects with neovascular age-related macular degeneration (nAMD) was carried out with recombinant human vascular endothelial growth factor receptor (VEGFR)-antibody-human complement receptor 1 (CR1) fusion protein Efdamrofuspalfa injection has reached the primary endpoint. IBI302 provides stable visual benefits and improved anatomical efficacy at long intervals (3 months and more), and potential improvements in macular atrophy have been observed. Next, Cinda Biotech will further explore the efficacy and safety of high-dose IBI302 administered at long intervals in the Phase III clinical study STAR. Progress of domestic new drug research and development (partial)
05 Domestic policies and regulations in the field of new drug research and development
No relevant policies and regulations have been released this week.
06 Hot news in the field of domestic new drug research and development
New Norway's rebirth journey
三十六计中,有一计暗渡陈仓,非常精彩。 去年下半年开始,新诺威一顿猛如虎的操作吸引了市场的极大关注,然而在如此高调之下,隐藏的却是石药集团诸多野心。 的确,商场如战场,如何以最小的代价盘活手中筹码,如何在危急时刻还能将上一军,需要足够的智慧和谋略。 经历数十年的风雨,石药集团最不缺的就是智慧与谋略,通过一系列运筹帷幄,再次给市场上了一课。 如今陈仓已然暗渡成功,胜利或许只是时间问题而已。 更多资讯,请阅读原文
Please contact the author for consultation and cooperation
Little D has something to say
In order to facilitate readers to read and save, we have compiled the original text of the weekly report into a PDF version. To obtain the full text, you can click on the blue word at the top and reply to "0328 Innovative Drug Weekly" on the background of the Yaodu Daily public account to download it.
